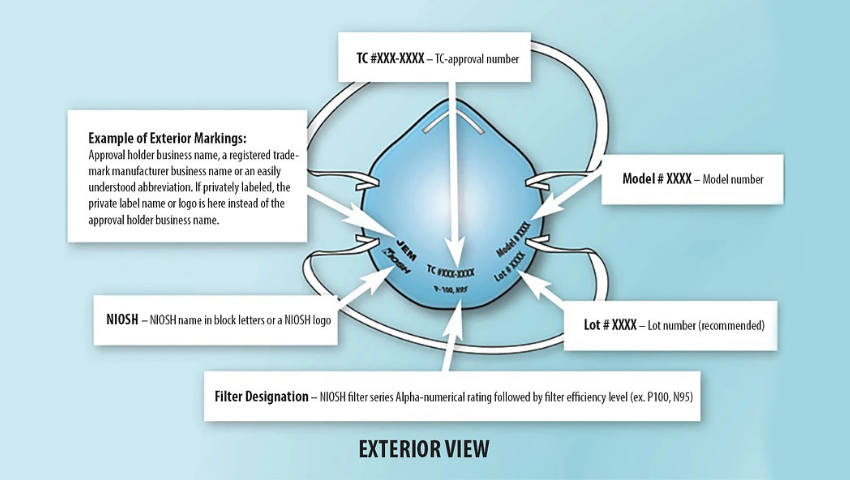
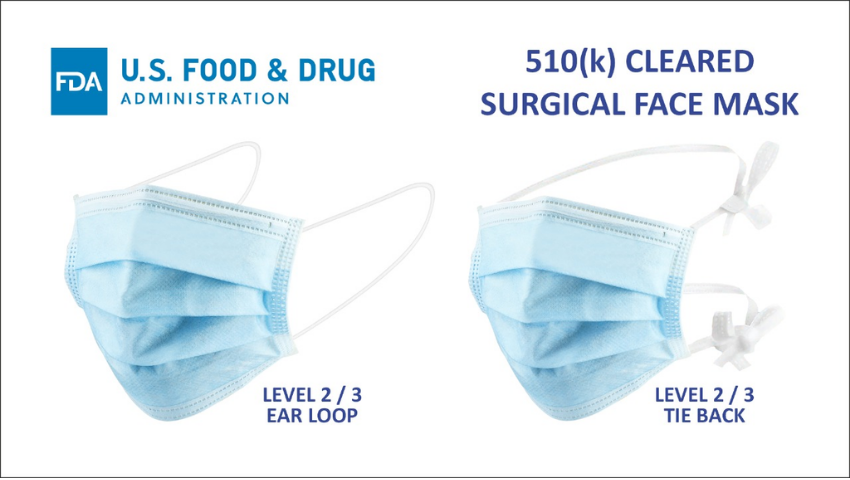
In the ever-evolving landscape of the pharmaceutical industry, compliance with regulatory guidelines is not merely a box to check but a fundamental aspect that ensures the safety of both employees and the products they develop. One critical area that has gained increased attention, especially in the wake of global health concerns, is mask usage within pharmaceutical companies. This blog will delve into the importance of compliance with mask regulations in the pharmaceutical sector and provide insights into navigating the complex web of guidelines.
The pharmaceutical industry operates under stringent regulatory frameworks to guarantee the quality and safety of pharmaceutical products. While guidelines may vary across regions, adherence to health and safety standards is universal. In recent years, the emphasis on respiratory hygiene, especially in the context of infectious diseases, has led to a reevaluation of mask-wearing protocols within pharmaceutical facilities.
Organizations like the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) play pivotal roles in shaping international health standards. Their recommendations often influence local regulatory bodies and set the tone for best practices in the industry. Pharmaceutical companies must stay abreast of these recommendations to align their mask usage policies with global health standards.
Pharmaceutical companies deal with a wide range of chemical compounds, some of which may pose respiratory hazards. Masks act as a crucial line of defense, protecting employees from inhaling harmful substances. Compliance with mask usage guidelines is, therefore, not only a regulatory requirement but a fundamental aspect of ensuring a safe working environment.
In addition to safeguarding employees, masks play a pivotal role in preserving the integrity of pharmaceutical products. Contamination risks, especially in cleanroom environments, are significantly reduced when employees adhere to proper mask protocols. This is paramount in industries where even the slightest contamination can compromise the quality of products and, subsequently, patient safety.
Pharmaceutical companies must develop customized mask usage protocols that align with their specific operations and the nature of their products. This involves a thorough risk assessment to identify potential respiratory hazards and tailor mask guidelines accordingly. The customization of protocols not only ensures compliance but also enhances overall safety measures.
Effective implementation of mask protocols requires comprehensive employee training. This includes education on the types of masks suitable for different scenarios, proper donning and doffing procedures, and the importance of consistent adherence. Regular training sessions and updates ensure that employees remain informed and vigilant about the evolving regulatory landscape.
Regulatory guidelines, especially those related to health and safety, are subject to change. Pharmaceutical companies must establish robust systems for continuous monitoring of regulatory updates. This proactive approach allows companies to adapt their mask protocols swiftly, ensuring compliance with the latest standards and regulations.
In the pharmaceutical industry, our unwavering commitment to compliance with mask usage guidelines is more than a regulatory mandate—it is a promise to prioritize the well-being of our dedicated workforce and uphold the integrity of our groundbreaking products. As we navigate the intricate regulatory landscape, Magnum Health & Safety, a renowned manufacturer of respiratory protection solutions, including our flagship products—the Magnum Surgical Masks and Magnum N95 Masks—plays a pivotal role in achieving these critical objectives.
Magnum Health & Safety understands that respiratory protection is not a one-size-fits-all solution. With precision and care, we manufacture high-quality, industry-compliant masks that are indispensable in pharmaceutical settings. Our dedication goes beyond crafting exceptional products; we aim to be active collaborators with pharmaceutical companies, working in tandem to customize mask solutions that align seamlessly with specific needs and operational nuances.
In recognizing the dynamic nature of the pharmaceutical industry, Magnum Health & Safety offers more than just masks; we offer a partnership that prioritizes continuous support. Our expertise empowers us to assist in creating and adapting personalized protocols, ensuring that Magnum’s Surgical Masks and N95 Masks integrate seamlessly with ever-evolving regulatory guidelines.
In the face of a fast-paced and ever-changing regulatory landscape, having Magnum Health & Safety as your partner provides a competitive advantage. We don’t just deliver respiratory protection; we provide ongoing support, keeping you informed about the latest regulatory updates and offering training resources to guarantee the proper implementation of our Magnum Masks—Surgical and N95.
Our collaboration stands as a testament to shared dedication to the highest standards of safety and compliance. As we collectively navigate the challenges of the pharmaceutical industry, Magnum Health & Safety, with its distinguished Surgical and N95 Masks, is not just a manufacturer but a committed ally, contributing to the creation of a safer and more resilient working environment for your employees. Together, we enhance our industry’s capacity to deliver innovative and safe products, ensuring a healthier future for all.